UCLA stem cell researchers use gene therapy to restore immune systems in 'bubble babies'
Public release date: 11-Sep-2012 [ | E-mail | Share ]
Contact: Kim Irwin kirwin@mednet.ucla.edu 310-435-9457 University of California - Los Angeles Health Sciences
UCLA stem cell researchers have found that a gene therapy regimen can safely restore immune systems to children with so-called "Bubble Boy" disease, a life threatening condition that if left untreated can be fatal within one to two years.
In the 11-year study, researchers were able to test two therapy regimens for 10 children with ADA-deficient severe combined immunodeficiency (SCID). During the study, they refined their approach to include a light dose of chemotherapy to help remove many of the blood stem cells in the bone marrow that are not creating an enzyme called adenosine deaminase (ADA), which is critical for the production and survival of healthy white blood cells, said study senior Dr. Donald Kohn, a professor of pediatrics and of microbiology, immunology, and molecular genetics in Life Sciences and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
The refined gene therapy and chemotherapy regimen proved superior to the other method tested in the study, restoring immune function to three of the six children who received it, Kohn said. Going forward, an even further refined regimen using a different type of virus delivery system will be studied in the next phase of the study, which already has enrolled eight of the 10 patients needed.
The study appears Aug. 30 in the advance online issue of the peer-reviewed journal Blood.
"We were very happy that in the human trials we were able to see a benefit in the patients after we modified the protocol," Kohn said. "Doctors treating ADA-deficient SCID have had too few options for too long, and we hope this will provide them with an efficient and effective treatment for this devastating disease."
Children born with SCID, an inherited immunodeficiency, are generally diagnosed at about six months. They are extremely vulnerable to infectious diseases and don't grow well. Chronic diarrhea, ear infections, recurrent pneumonia and profuse oral candidiasis commonly occur in these children. SCID cases occur in about 1 of 100,000 births
Currently, the only treatment for ADA-deficient SCID calls for injecting the patients twice a week with the necessary enzyme, Kohn said, a life-long process that is very expensive and often doesn't return the immune system to optimal levels. These patients also can undergo bone marrow transplants from matched siblings, but matches can be very rare.
About 15 percent of all SCID patients are ADA-deficient. Kohn and his team used a virus delivery system that he had developed in his lab in the 1990s to restore the gene that produces the missing enzyme necessary for a healthy immune system. To date, about 40 children with SCID have received gene therapy in clinical trials around the world, Kohn said.
Read more from the original source:
UCLA stem cell researchers use gene therapy to restore immune systems in 'bubble babies'
Recommendation and review posted by Bethany Smith
Stem cell researchers use gene therapy to restore immune systems in 'Bubble Boy' disease
ScienceDaily (Sep. 11, 2012) UCLA stem cell researchers have found that a gene therapy regimen can safely restore immune systems to children with so-called "Bubble Boy" disease, a life threatening condition that if left untreated can be fatal within one to two years.
In the 11-year study, researchers were able to test two therapy regimens for 10 children with ADA-deficient severe combined immunodeficiency (SCID). During the study, they refined their approach to include a light dose of chemotherapy to help remove many of the blood stem cells in the bone marrow that are not creating an enzyme called adenosine deaminase (ADA), which is critical for the production and survival of healthy white blood cells, said study senior Dr. Donald Kohn, a professor of pediatrics and of microbiology, immunology, and molecular genetics in Life Sciences and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
The refined gene therapy and chemotherapy regimen proved superior to the other method tested in the study, restoring immune function to three of the six children who received it, Kohn said. Going forward, an even further refined regimen using a different type of virus delivery system will be studied in the next phase of the study, which already has enrolled eight of the 10 patients needed.
The study appears Aug. 30 in the advance online issue of the peer-reviewed journal Blood.
"We were very happy that in the human trials we were able to see a benefit in the patients after we modified the protocol," Kohn said. "Doctors treating ADA-deficient SCID have had too few options for too long, and we hope this will provide them with an efficient and effective treatment for this devastating disease."
Children born with SCID, an inherited immunodeficiency, are generally diagnosed at about six months. They are extremely vulnerable to infectious diseases and don't grow well. Chronic diarrhea, ear infections, recurrent pneumonia and profuse oral candidiasis commonly occur in these children. SCID cases occur in about 1 of 100,000 births
Currently, the only treatment for ADA-deficient SCID calls for injecting the patients twice a week with the necessary enzyme, Kohn said, a life-long process that is very expensive and often doesn't return the immune system to optimal levels. These patients also can undergo bone marrow transplants from matched siblings, but matches can be very rare.
About 15 percent of all SCID patients are ADA-deficient. Kohn and his team used a virus delivery system that he had developed in his lab in the 1990s to restore the gene that produces the missing enzyme necessary for a healthy immune system. To date, about 40 children with SCID have received gene therapy in clinical trials around the world, Kohn said.
Two slightly different viral vectors were tested in the study, each modified to deliver healthy ADA genes into the bone marrow cells of the patients so the needed enzyme could be produced and make up for the cells that don't have the gene. Four of the 10 patients in the study remained on their enzyme replacement therapy during the gene therapy study. There were no side effects, but their immune systems were not sufficiently restored, Kohn said.
In the next six patients, the enzyme therapy was stopped and a small dose of chemotherapy was given before starting the gene therapy to deplete the ADA-deficient stem cells in their bone marrow. Of those patients, half had their immune systems restored. The human findings confirmed another study, also published recently in Blood by Kohn and UCLA colleague Dr. Denise Carbonaro-Sarracino, which tested the techniques in parallel, using a mouse model of ADA-deficient SCID.
The rest is here:
Stem cell researchers use gene therapy to restore immune systems in 'Bubble Boy' disease
Recommendation and review posted by Bethany Smith
NIH researchers restore children's immune systems with refinements in gene therapy
Public release date: 11-Sep-2012 [ | E-mail | Share ]
Contact: Raymond MacDougall macdougallr@mail.nih.gov 301-402-0911 NIH/National Human Genome Research Institute
Researchers have demonstrated that a refined gene therapy approach safely restores the immune systems of some children with severe combined immunodeficiency (SCID). The rare condition blocks the normal development of a newborn's immune system, leaving the child susceptible to every passing microbe. Children with SCID experience chronic infections, which usually triggers the diagnosis. Their lifespan is two years if doctors cannot restore their immunity.
The findings from facilities including the National Institutes of Health, the University of California, Los Angeles (UCLA), and the Children's Hospital Los Angeles, are reported in the Sept. 11, 2012, advanced online issue of the journal Blood, the official journal of the American Society of Hematology.
In the 11-year study, the researchers tested a combination of techniques for gene therapy, arriving at one that produced normal levels of immune function for three patients.
"Doctors who treat patients with SCID have had limited treatment options for too long," said Dan Kastner, M.D., Ph.D., scientific director of the National Human Genome Research Institute (NHGRI), part of the NIH. "The research teams and the patients who have participated in the studies have together achieved an impressive advance toward a cure that is welcome news for both the scientific and patient communities."
Gene therapy is an experimental method for treating patients with genetic diseases. It is intended to integrate functioning genes among those naturally existing in the cells of the body to make up for faulty genes. Researchers in the current study tested a set of methods to improve outcomes for children with a particular form of SCID.
"This is a highly rewarding study for those of us in the clinic and lab," said Fabio Candotti, M.D., a senior author and a senior investigator in NHGRI's Genetics and Molecular Biology Branch. "Not only have we realized an important advancement in gene therapy, but we have seen a renewal of health in our patients."
While rare, SCID became widely known because of the remarkable boy-in-the-bubble story of the 1970s. The story was based in part on a boy named David Vetter, who lived for 13 years in a plastic isolation unit to protect him from infections. He died following an unsuccessful bone marrow transplant that doctors had hoped would repair his immune system.
SCID has many causes. In one type, a gene that produces the adenosine deaminase (ADA) enzyme becomes mutated and fails to produce the normal enzyme. Without ADA, a chemically altered form of adenosine, one of DNA's building blocks, accumulates in rapidly dividing bone marrow cells, killing them and destroying the immune system in the process. Normal bone marrow makes healthy white blood cells, or lymphocytes, which are the key players in the immune response that reacts against harmful bacteria and destroys cells infected by viruses. ADA deficiency accounts for some 15 percent of SCID cases.
Visit link:
NIH researchers restore children's immune systems with refinements in gene therapy
Recommendation and review posted by Bethany Smith
Researchers improve gene therapy technique for children with immune disorder
Public release date: 11-Sep-2012 [ | E-mail | Share ]
Contact: Claire Gwayi-Chore cgwayi-chore@hematology.org 202-776-0544 American Society of Hematology
By including chemotherapy as a conditioning regimen prior to treatment, researchers have developed a refined gene therapy approach that safely and effectively restores the immune system of children with a form of severe combined immunodeficiency (SCID), according to a study published online today in Blood, the Journal of the American Society of Hematology (ASH).
SCID is a group of rare and debilitating genetic disorders that affect the normal development of the immune system in newborns. Infants with SCID are prone to serious, life-threatening infections within the first few months of life and require extensive treatment for survival beyond infancy.
Adenosine deaminase (ADA) deficiency, which accounts for approximately 15 percent of all SCID cases, develops when a gene mutation prohibits the production of ADA, an enzyme that breaks down toxic molecules that can accumulate to harmful levels and kill lymphocytes, the specialized white blood cells that help make up the immune system. In its absence, infants with ADA-deficient SCID lack almost all immune defenses and their condition is almost always fatal within two years if left untreated. Standard treatment for ADA-deficient SCID is a hematopoietic stem cell transplant (HSCT) from a sibling or related donor; however, finding a matched donor can be difficult and transplants can carry significant risks. An alternate treatment method, enzyme replacement therapy (ERT), involves regular injections of the ADA enzyme to maintain the immune system and can help restore immune function; however, the treatments are extremely expensive and painful for the young patients and the effects are often only temporary.
Given the limitations of HSCT and ERT, in the 1990s researchers began investigating the efficacy of gene therapy for ADA-deficient SCID. They discovered that they could "correct" the function of a mutated gene by adding a healthy copy into the cells of the body that help fight infectious diseases. Since then, there have been significant advances in gene therapy for SCID, yet successful gene therapy in patients with ADA-deficient SCID has been seen in only a small series of children due to the difficulty of introducing a healthy ADA gene into bone marrow stem cells and to engraft these cells back into the patients.
"Although the basic steps of gene therapy for patients with SCID have been known for a while, technical and clinical challenges still exist and we wanted to find an optimized gene therapy protocol to restore immunity for young children with ADA-deficient SCID," said Fabio Candotti, MD, one of the study's senior authors, senior investigator in the Genetics and Molecular Biology Branch of the National Human Genome Research Institute at the National Institutes of Health, and chair of the ASH Scientific Committee on Immunology and Host Defense.
To determine whether an enhanced gene therapy approach would improve immunity in children with ADA-deficient SCID, the teams of Dr. Candotti and Donald B. Kohn, MD, director of the Human Gene Medicine Program at the University of California, Los Angeles (UCLA), Professor of Pediatrics and of Microbiology, Immunology, and Molecular Genetics, and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, conducted a clinical trial in 10 patients with the disorder. For the first time, Drs. Candotti and Kohn and their team of investigators compared two different retroviral vectors, MND-ADA and GCsapM-ADA, to transport normal ADA genes into the young patients' bone marrow stem cells as well as two different treatment plans in preparation for receiving gene therapy. Following therapy, investigators found that more bone marrow stem cells were marked with the MND-ADA vector, demonstrating its superiority over the GCsapM-ADA vector.
The investigators also sought to determine whether providing a low dose of chemotherapy prior to gene therapy, known as a pre-transplant conditioning regimen, would successfully deplete the young patients' bone marrow stem cells and make room for gene-corrected stem cells. In four patients, gene therapy was performed without chemotherapy, and the patients remained on ERT throughout the entire procedure to evaluate the efficiency of ERT combined with gene therapy. While these patients did not experience any adverse effects, they also did not experience a significant increase in their levels of the ADA enzyme. They also maintained low absolute lymphocyte counts (ALC) and minimal immune system function, leading the researchers to believe that ERT may weaken the therapy's effect by diluting the number of gene-corrected lymphocytes.
The remaining six patients were treated with the chemotherapy drug busulfan prior to gene therapy and ERT was discontinued prior to the gene therapy procedure. A significant increase in ADA was observed in all six patients; half of them remain off of ERT with partial immune reconstitution findings that support results from prior trials in Italy and the United Kingdom using chemotherapy prior to gene therapy and discontinuting ERT. While the ALC of all six patients declined sharply in the first few months due to combined effects of busulfan administration and ERT withdrawal, their counts increased from six to 24 months, even in the three patients that remained off of ERT. After adjusting the chemotherapy dosage, investigators were able to determine an optimal level for enhancing the efficacy of the gene-therapy-corrected cells with minimal toxicity.
See the original post:
Researchers improve gene therapy technique for children with immune disorder
Recommendation and review posted by Bethany Smith
Gene therapy technique for children with immune disorder improved
ScienceDaily (Sep. 11, 2012) By including chemotherapy as a conditioning regimen prior to treatment, researchers have developed a refined gene therapy approach that safely and effectively restores the immune system of children with a form of severe combined immunodeficiency (SCID), according to a study published online September 11 in Blood, the Journal of the American Society of Hematology (ASH).
SCID is a group of rare and debilitating genetic disorders that affect the normal development of the immune system in newborns. Infants with SCID are prone to serious, life-threatening infections within the first few months of life and require extensive treatment for survival beyond infancy.
Adenosine deaminase (ADA) deficiency, which accounts for approximately 15 percent of all SCID cases, develops when a gene mutation prohibits the production of ADA, an enzyme that breaks down toxic molecules that can accumulate to harmful levels and kill lymphocytes, the specialized white blood cells that help make up the immune system. In its absence, infants with ADA-deficient SCID lack almost all immune defenses and their condition is almost always fatal within two years if left untreated. Standard treatment for ADA-deficient SCID is a hematopoietic stem cell transplant (HSCT) from a sibling or related donor; however, finding a matched donor can be difficult and transplants can carry significant risks. An alternate treatment method, enzyme replacement therapy (ERT), involves regular injections of the ADA enzyme to maintain the immune system and can help restore immune function; however, the treatments are extremely expensive and painful for the young patients and the effects are often only temporary.
Given the limitations of HSCT and ERT, in the 1990s researchers began investigating the efficacy of gene therapy for ADA-deficient SCID. They discovered that they could "correct" the function of a mutated gene by adding a healthy copy into the cells of the body that help fight infectious diseases. Since then, there have been significant advances in gene therapy for SCID, yet successful gene therapy in patients with ADA-deficient SCID has been seen in only a small series of children due to the difficulty of introducing a healthy ADA gene into bone marrow stem cells and to engraft these cells back into the patients.
"Although the basic steps of gene therapy for patients with SCID have been known for a while, technical and clinical challenges still exist and we wanted to find an optimized gene therapy protocol to restore immunity for young children with ADA-deficient SCID," said Fabio Candotti, MD, one of the study's senior authors, senior investigator in the Genetics and Molecular Biology Branch of the National Human Genome Research Institute at the National Institutes of Health, and chair of the ASH Scientific Committee on Immunology and Host Defense.
To determine whether an enhanced gene therapy approach would improve immunity in children with ADA-deficient SCID, the teams of Dr. Candotti and Donald B. Kohn, MD, director of the Human Gene Medicine Program at the University of California, Los Angeles (UCLA), Professor of Pediatrics and of Microbiology, Immunology, and Molecular Genetics, and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA, conducted a clinical trial in 10 patients with the disorder. For the first time, Drs. Candotti and Kohn and their team of investigators compared two different retroviral vectors, MND-ADA and GCsapM-ADA, to transport normal ADA genes into the young patients' bone marrow stem cells as well as two different treatment plans in preparation for receiving gene therapy. Following therapy, investigators found that more bone marrow stem cells were marked with the MND-ADA vector, demonstrating its superiority over the GCsapM-ADA vector.
The investigators also sought to determine whether providing a low dose of chemotherapy prior to gene therapy, known as a pre-transplant conditioning regimen, would successfully deplete the young patients' bone marrow stem cells and make room for gene-corrected stem cells. In four patients, gene therapy was performed without chemotherapy, and the patients remained on ERT throughout the entire procedure to evaluate the efficiency of ERT combined with gene therapy. While these patients did not experience any adverse effects, they also did not experience a significant increase in their levels of the ADA enzyme. They also maintained low absolute lymphocyte counts (ALC) and minimal immune system function, leading the researchers to believe that ERT may weaken the therapy's effect by diluting the number of gene-corrected lymphocytes.
The remaining six patients were treated with the chemotherapy drug busulfan prior to gene therapy and ERT was discontinued prior to the gene therapy procedure. A significant increase in ADA was observed in all six patients; half of them remain off of ERT with partial immune reconstitution -- findings that support results from prior trials in Italy and the United Kingdom using chemotherapy prior to gene therapy and discontinuting ERT. While the ALC of all six patients declined sharply in the first few months due to combined effects of busulfan administration and ERT withdrawal, their counts increased from six to 24 months, even in the three patients that remained off of ERT. After adjusting the chemotherapy dosage, investigators were able to determine an optimal level for enhancing the efficacy of the gene-therapy-corrected cells with minimal toxicity.
This study is the first to detail comparisons of ADA-deficient SCID patient outcomes between those treated with gene therapy who have not received pre-transplant conditioning while continuing to receive ERT with those receiving pre-transplant conditioning without the administration of ERT. This study is also the first to compare two different viral vectors to transport normal ADA genes into patient bone marrow.
"We were very happy that in this trial we were able to see a benefit in the patients after we modified the protocol," said Dr. Kohn. "Doctors treating ADA-deficient SCID have had too few options for too long, and we hope this will provide them with an efficient and effective treatment for this devastating disease."
Read the rest here:
Gene therapy technique for children with immune disorder improved
Recommendation and review posted by Bethany Smith
Stem cell researchers use gene therapy to restore immune systems in ‘Bubble Boy’ disease
ScienceDaily (Sep. 11, 2012) UCLA stem cell researchers have found that a gene therapy regimen can safely restore immune systems to children with so-called "Bubble Boy" disease, a life threatening condition that if left untreated can be fatal within one to two years.
In the 11-year study, researchers were able to test two therapy regimens for 10 children with ADA-deficient severe combined immunodeficiency (SCID). During the study, they refined their approach to include a light dose of chemotherapy to help remove many of the blood stem cells in the bone marrow that are not creating an enzyme called adenosine deaminase (ADA), which is critical for the production and survival of healthy white blood cells, said study senior Dr. Donald Kohn, a professor of pediatrics and of microbiology, immunology, and molecular genetics in Life Sciences and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
The refined gene therapy and chemotherapy regimen proved superior to the other method tested in the study, restoring immune function to three of the six children who received it, Kohn said. Going forward, an even further refined regimen using a different type of virus delivery system will be studied in the next phase of the study, which already has enrolled eight of the 10 patients needed.
The study appears Aug. 30 in the advance online issue of the peer-reviewed journal Blood.
"We were very happy that in the human trials we were able to see a benefit in the patients after we modified the protocol," Kohn said. "Doctors treating ADA-deficient SCID have had too few options for too long, and we hope this will provide them with an efficient and effective treatment for this devastating disease."
Children born with SCID, an inherited immunodeficiency, are generally diagnosed at about six months. They are extremely vulnerable to infectious diseases and don't grow well. Chronic diarrhea, ear infections, recurrent pneumonia and profuse oral candidiasis commonly occur in these children. SCID cases occur in about 1 of 100,000 births
Currently, the only treatment for ADA-deficient SCID calls for injecting the patients twice a week with the necessary enzyme, Kohn said, a life-long process that is very expensive and often doesn't return the immune system to optimal levels. These patients also can undergo bone marrow transplants from matched siblings, but matches can be very rare.
About 15 percent of all SCID patients are ADA-deficient. Kohn and his team used a virus delivery system that he had developed in his lab in the 1990s to restore the gene that produces the missing enzyme necessary for a healthy immune system. To date, about 40 children with SCID have received gene therapy in clinical trials around the world, Kohn said.
Two slightly different viral vectors were tested in the study, each modified to deliver healthy ADA genes into the bone marrow cells of the patients so the needed enzyme could be produced and make up for the cells that don't have the gene. Four of the 10 patients in the study remained on their enzyme replacement therapy during the gene therapy study. There were no side effects, but their immune systems were not sufficiently restored, Kohn said.
In the next six patients, the enzyme therapy was stopped and a small dose of chemotherapy was given before starting the gene therapy to deplete the ADA-deficient stem cells in their bone marrow. Of those patients, half had their immune systems restored. The human findings confirmed another study, also published recently in Blood by Kohn and UCLA colleague Dr. Denise Carbonaro-Sarracino, which tested the techniques in parallel, using a mouse model of ADA-deficient SCID.
Read more here:
Stem cell researchers use gene therapy to restore immune systems in 'Bubble Boy' disease
Recommendation and review posted by simmons
UCLA stem cell researchers use gene therapy to restore immune systems in ‘bubble babies’
Public release date: 11-Sep-2012 [ | E-mail | Share ]
Contact: Kim Irwin kirwin@mednet.ucla.edu 310-435-9457 University of California - Los Angeles Health Sciences
UCLA stem cell researchers have found that a gene therapy regimen can safely restore immune systems to children with so-called "Bubble Boy" disease, a life threatening condition that if left untreated can be fatal within one to two years.
In the 11-year study, researchers were able to test two therapy regimens for 10 children with ADA-deficient severe combined immunodeficiency (SCID). During the study, they refined their approach to include a light dose of chemotherapy to help remove many of the blood stem cells in the bone marrow that are not creating an enzyme called adenosine deaminase (ADA), which is critical for the production and survival of healthy white blood cells, said study senior Dr. Donald Kohn, a professor of pediatrics and of microbiology, immunology, and molecular genetics in Life Sciences and a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.
The refined gene therapy and chemotherapy regimen proved superior to the other method tested in the study, restoring immune function to three of the six children who received it, Kohn said. Going forward, an even further refined regimen using a different type of virus delivery system will be studied in the next phase of the study, which already has enrolled eight of the 10 patients needed.
The study appears Aug. 30 in the advance online issue of the peer-reviewed journal Blood.
"We were very happy that in the human trials we were able to see a benefit in the patients after we modified the protocol," Kohn said. "Doctors treating ADA-deficient SCID have had too few options for too long, and we hope this will provide them with an efficient and effective treatment for this devastating disease."
Children born with SCID, an inherited immunodeficiency, are generally diagnosed at about six months. They are extremely vulnerable to infectious diseases and don't grow well. Chronic diarrhea, ear infections, recurrent pneumonia and profuse oral candidiasis commonly occur in these children. SCID cases occur in about 1 of 100,000 births
Currently, the only treatment for ADA-deficient SCID calls for injecting the patients twice a week with the necessary enzyme, Kohn said, a life-long process that is very expensive and often doesn't return the immune system to optimal levels. These patients also can undergo bone marrow transplants from matched siblings, but matches can be very rare.
About 15 percent of all SCID patients are ADA-deficient. Kohn and his team used a virus delivery system that he had developed in his lab in the 1990s to restore the gene that produces the missing enzyme necessary for a healthy immune system. To date, about 40 children with SCID have received gene therapy in clinical trials around the world, Kohn said.
Continued here:
UCLA stem cell researchers use gene therapy to restore immune systems in 'bubble babies'
Recommendation and review posted by simmons
University of Maryland study: Neonatal heart stem cells may help mend kids' broken hearts
Public release date: 10-Sep-2012 [ | E-mail | Share ]
Contact: Bill Seiler bseiler@umm.edu 410-328-8919 University of Maryland Medical Center
Baltimore, MD September 10, 2012 Researchers at the University of Maryland School of Medicine, who are exploring novel ways to treat serious heart problems in children, have conducted the first direct comparison of the regenerative abilities of neonatal and adult-derived human cardiac stem cells. Among their findings: cardiac stem cells (CSCs) from newborns have a three-fold ability to restore heart function to nearly normal levels compared with adult CSCs. Further, in animal models of heart attack, hearts treated with neonatal stem cells pumped stronger than those given adult cells. The study is published in the September 11, 2012, issue of Circulation.
"The surprising finding is that the cells from neonates are extremely regenerative and perform better than adult stem cells," says the study's senor author, Sunjay Kaushal, M.D., Ph.D., associate professor of surgery at the University of Maryland School of Medicine and director, pediatric cardiac surgery at the University of Maryland Medical Center. "We are extremely excited and hopeful that this new cell-based therapy can play an important role in the treatment of children with congenital heart disease, many of whom don't have other options."
Dr. Kaushal envisions cellular therapy as either a stand-alone therapy for children with heart failure or an adjunct to medical and surgical treatments. While surgery can provide structural relief for some patients with congenital heart disease and medicine can boost heart function up to two percent, he says cellular therapy may improve heart function even more dramatically. "We're looking at this type of therapy to improve heart function in children by 10, 12, or 15 percent. This will be a quantum leap in heart function improvement."
Heart failure in children, as in adults, has been on the rise in the past decade and the prognosis for patients hospitalized with heart failure remains poor. In contrast to adults, Dr. Kaushal says heart failure in children is typically the result of a constellation of problems: reduced cardiac blood flow; weakening and enlargement of the heart; and various congenital malformations. Recent research has shown that several types of cardiac stem cells can help the heart repair itself, essentially reversing the theory that a broken heart cannot be mended.
Stem cells are unspecialized cells that can become tissue- or organ-specific cells with a particular function. In a process called differentiation, cardiac stem cells may develop into rhythmically contracting muscle cells, smooth muscle cells or endothelial cells. Stem cells in the heart may also secrete growth factors conducive to forming heart muscle and keeping the muscle from dying.
To conduct the study, researchers obtained a small amount of heart tissue during normal cardiac surgery from 43 neonates and 13 adults. The cells were expanded in a growth medium yielding millions of cells. The researchers developed a consistent way to isolate and grow neonatal stem cells from as little as 20 milligrams of heart tissue. Adult and neonate stem cell activity was observed both in the laboratory and in animal models. In addition, the animal models were compared to controls that were not given the stem cells.
Dr. Kaushal says it is not clear why the neonatal stem cells performed so well. One explanation hinges on sheer numbers: there are many more stem cells in a baby's heart than in the adult heart. Another explanation: neonate-derived cells release more growth factors that trigger blood vessel development and/or preservation than adult cells.
"This research provides an important link in our quest to understand how stem cells function and how they can best be applied to cure disease and correct medical deficiencies," says E. Albert Reece, M.D., Ph.D., M.B.A., vice president for medical affairs, University of Maryland; the John Z. and Akiko K. Bowers Distinguished Professor; and dean, University of Maryland School of Medicine. "Sometimes simple science is the best science. In this case, a basic, comparative study has revealed in stark terms the powerful regenerative qualities of neonatal cardiac stem cells, heretofore unknown."
Read more here:
University of Maryland study: Neonatal heart stem cells may help mend kids' broken hearts
Recommendation and review posted by Bethany Smith
Cancer-Causing Gene Alone Doesn't Trigger Pancreatic Cancer
MULTIMEDIA ALERT: Video resources, including an interview with Dr. Crawford, are available for journalists at the Mayo Clinic News Network.
Newswise JACKSONVILLE, Fla. More than a cancer-causing gene is needed to trigger pancreatic cancer, a study led by Mayo Clinic has found. A second factor creates a perfect storm that allows tumors to form, the researchers say. The study, published in the Sept. 10 issue of Cancer Cell, overturns the current belief that a mutation in the KRAS oncogene is enough to initiate pancreatic cancer and unrestrained cell growth.
The findings uncover critical clues on how pancreatic cancer develops and why few patients benefit from current therapies. The findings also provide ideas about how to improve treatment and prevention of pancreatic cancer.
The research team, led by Howard C. Crawford, Ph.D., a cancer biologist at Mayo Clinics campus in Florida, and Jens Siveke, M.D., at Technical University in Munich, Germany, found that for pancreatic cancer to form, mutated KRAS must recruit a second player: the epidermal growth factor receptor, or EGFR.A third genetic participant known as Trp53 makes pancreatic tumors very difficult to treat, the study showed.
The scientists also found that EGFR was required in pancreatic cancer initiated by pancreatic inflammation known as pancreatitis.
We believe the perfect storm needed to trigger pancreatic cancer include KRAS mutations and inflammation in the organ, which then work synergistically to turn on EGFR, says Dr. Crawford.
The bottom line is, without EGFR, tumors dont form and that was never known before this study, he says. We also think that inflammation in the pancreas has a big impact on turning on EGFR.
The researchers discovered that when they blocked EGFR activity, the mice studied were protected against developing chronic pancreatitis and pancreatic cancer.
They further found that in mice that had lost expression of the TP53 tumor suppressor a situation that mirrors up to 60 percent of human pancreatic cancer cases tumors escape the dependency on EGFR for initiation and continued growth of pancreatic cancer, Dr. Crawford says.
Pancreatic cancer is a highly lethal disease; no drug has been able to target the mutant KRAS protein. The study suggests some patients, such as those with chronic pancreatitis, may be good candidates for treatment with EGFR inhibitors to fight or prevent pancreatic cancer, Dr. Crawford says.
Read the original here:
Cancer-Causing Gene Alone Doesn't Trigger Pancreatic Cancer
Recommendation and review posted by Bethany Smith
University of Maryland Study Suggests Neonatal Cardiac Stem Cells May Help Mend Children's Broken Hearts
Cardiac stem cells from newborns show stronger regenerative ability than adult stem cells
BALTIMORE, Sept. 10, 2012 /PRNewswire-USNewswire/ -- Researchers at the University of Maryland School of Medicine, who are exploring novel ways to treat serious heart problems in children, have conducted the first direct comparison of the regenerative abilities of neonatal and adult-derived human cardiac stem cells. Among their findings: cardiac stem cells (CSCs) from newborns have a three-fold ability to restore heart function to nearly normal levels compared with adult CSCs. Further, in animal models of heart attack, hearts treated with neonatal stem cells pumped stronger than those given adult cells. The study is published in the September 11, 2012, issue of Circulation.
"The surprising finding is that the cells from neonates are extremely regenerative and perform better than adult stem cells," says the study's senor author, Sunjay Kaushal, M.D., Ph.D., associate professor of surgery at the University of Maryland School of Medicine and director, pediatric cardiac surgery at the University of Maryland Medical Center. "We are extremely excited and hopeful that this new cell-based therapy can play an important role in the treatment of children with congenital heart disease, many of whom don't have other options."
Dr. Kaushal envisions cellular therapy as either a stand-alone therapy for children with heart failure or an adjunct to medical and surgical treatments. While surgery can provide structural relief for some patients with congenital heart disease and medicine can boost heart function up to two percent, he says cellular therapy may improve heart function even more dramatically. "We're looking at this type of therapy to improve heart function in children by 10, 12, or 15 percent. This will be a quantum leap in heart function improvement."
Heart failure in children, as in adults, has been on the rise in the past decade and the prognosis for patients hospitalized with heart failure remains poor. In contrast to adults, Dr. Kaushal says heart failure in children is typically the result of a constellation of problems: reduced cardiac blood flow; weakening and enlargement of the heart; and various congenital malformations. Recent research has shown that several types of cardiac stem cells can help the heart repair itself, essentially reversing the theory that a broken heart cannot be mended.
Stem cells are unspecialized cells that can become tissue- or organ-specific cells with a particular function. In a process called differentiation, cardiac stem cells may develop into rhythmically contracting muscle cells, smooth muscle cells or endothelial cells. Stem cells in the heart may also secrete growth factors conducive to forming heart muscle and keeping the muscle from dying.
To conduct the study, researchers obtained a small amount of heart tissue during normal cardiac surgery from 43 neonates and 13 adults. The cells were expanded in a growth medium yielding millions of cells. The researchers developed a consistent way to isolate and grow neonatal stem cells from as little as 20 milligrams of heart tissue. Adult and neonate stem cell activity was observed both in the laboratory and in animal models. In addition, the animal models were compared to controls that were not given the stem cells.
Dr. Kaushal says it is not clear why the neonatal stem cells performed so well. One explanation hinges on sheer numbers: there are many more stem cells in a baby's heart than in the adult heart. Another explanation: neonate-derived cells release more growth factors that trigger blood vessel development and/or preservation than adult cells.
Go here to see the original:
University of Maryland Study Suggests Neonatal Cardiac Stem Cells May Help Mend Children's Broken Hearts
Recommendation and review posted by Bethany Smith
Soon a stem cell jabs to end wrinkles
London, Sep 10:
Ladies, you may not have to depend upon painful Botox injections and expensive cosmetic surgery for long to look young.
A British firm is trialling a new natural method which involves injecting the patients own stem cells to restore skins youthful elasticity.
Researchers believe they will spur the growth of new skin cells, called fibroblasts, which make the elastic ingredient collagen which is produced in large quantities when we are young, but declines as we age, the Daily Mail reported.
The company Pharmacells, based in Glasgow, plans to begin clinical trials in 12 months, using stem cells harvested from a blood sample from the patients.
They believe the procedure could be commercially available in just three years, potentially revolutionising the market for anti-ageing treatments.
By using the bodys own cells, it is billed as a more natural approach to reducing the signs of ageing than Botox, a chemical which freezes the facial muscles to smooth wrinkles.
The company has licensed the technology to harvest a new type of stem cell called a blastomere-like stem cell (CORR) which is found circulating in the blood.
Like other types of stem cells, it is unspecialised and can develop into many other types of cell in the human body such as a liver, brain or skin cell.
The advantage of this particular one it is available in very large doses from one blood sample.
Originally posted here:
Soon a stem cell jabs to end wrinkles
Recommendation and review posted by Bethany Smith
Could this stem cell cure for wrinkles end the endless hunt for the perfect skin cream?
British firm is trialling new method by injecting patient's own stem cells to restore skin's youthful elasticity
By Tamara Cohen
PUBLISHED: 10:40 EST, 9 September 2012 | UPDATED: 02:12 EST, 10 September 2012
Scientists will begin clinical trials in 12 months, using stem cells harvested from a blood sample from the patients
Scientists are working on a new weapon in the war against wrinkles.
There are not many things women have not tried in the quest for a youthful complexion from lotions and potions to Botox and cosmetic surgery.
But a British firm is trialling a new method which involves injecting the patients own stem cells to restore skins youthful elasticity.
Researchers believe they will spur the growth of new skin cells, called fibroblasts, which make the elastic ingredient collagen which is produced in large quantities when we are young, but declines as we age.
The company Pharmacells, based in Glasgow, plan to begin clinical trials in 12 months, using stem cells harvested from a blood sample from the patients.
They believe the procedure could be commercially available in just three years, potentially revolutionising the market for anti-ageing treatments.
Read the original post:
Could this stem cell cure for wrinkles end the endless hunt for the perfect skin cream?
Recommendation and review posted by Bethany Smith
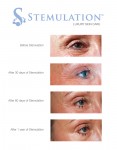
Introducing Canadians to a whole new way to treat aging skin: Stemulation
TORONTO, Sept. 10, 2012 /CNW/ - Sigmacon Skin Sciences announced it is the exclusive Canadian distributor of Stemulation, a luxury skin care line that uses the healing power of human stem cells to combat wrinkles and other signs of aging.
Stemulation is based on the science that stem cells can be effectively used for skin rejuvenation, tissue repair and wound healing. A research team of specialists spent two years capturing growth factors from adult human skin cells, which they turned into an active ingredient and the basis for Stemulation products. These growth factors stimulate collagen and the reproduction of new skin cells to reduce wrinkles, eliminate sun spots and smooth scars and fine lines. It truly is a groundbreaking (and technology-backed) new way to achieve younger-looking skin!
The Stemulation line includes a serum, cleanser, exfoliant and face and body creams. The line will be sold through select doctors, estheticians and medical spas.
ABOUT Sigmacon Skin Sciences is the national distributor of a comprehensive set of performance skin care products with dedicated product specialists and trainings all across Canada. Our product lines include professional treatments, sun protection products and results-oriented home care. Sigmacon is also the distributor of advanced medical and aesthetic devices. Visit http://www.skinsciences.ca to learn more.
Image with caption: "The Future of Skin Care: Stemulation Facial Serum and Boost Crme used over 1 year. (CNW Group/Sigmacon Skin Sciences)". Image available at: http://photos.newswire.ca/images/download/20120910_C3135_PHOTO_EN_17420.jpg
Link:
Introducing Canadians to a whole new way to treat aging skin: Stemulation
Recommendation and review posted by Bethany Smith
Cancer-causing gene alone doesn't trigger pancreatic cancer, Mayo-led study finds
Public release date: 10-Sep-2012 [ | E-mail | Share ]
Contact: Kevin Punsky punsky.kevin@mayo.edu 904-953-2299 Mayo Clinic
JACKSONVILLE, Fla. More than a cancer-causing gene is needed to trigger pancreatic cancer, a study led by Mayo Clinic has found. A second factor creates a "perfect storm" that allows tumors to form, the researchers say. The study, published in the Sept. 10 issue of Cancer Cell, overturns the current belief that a mutation in the KRAS oncogene is enough to initiate pancreatic cancer and unrestrained cell growth.
The findings uncover critical clues on how pancreatic cancer develops and why few patients benefit from current therapies. The findings also provide ideas about how to improve treatment and prevention of pancreatic cancer.
The research team, led by Howard C. Crawford, Ph.D., a cancer biologist at Mayo Clinic's campus in Florida, and Jens Siveke, M.D., at Technical University in Munich, Germany, found that for pancreatic cancer to form, mutated KRAS must recruit a second player: the epidermal growth factor receptor, or EGFR.A third genetic participant known as Trp53 makes pancreatic tumors very difficult to treat, the study showed.
The scientists also found that EGFR was required in pancreatic cancer initiated by pancreatic inflammation known as pancreatitis.
"We believe the perfect storm needed to trigger pancreatic cancer include KRAS mutations and inflammation in the organ, which then work synergistically to turn on EGFR," says Dr. Crawford.
"The bottom line is, without EGFR, tumors don't form and that was never known before this study," he says. "We also think that inflammation in the pancreas has a big impact on turning on EGFR."
The researchers discovered that when they blocked EGFR activity, the mice studied were protected against developing chronic pancreatitis and pancreatic cancer.
They further found that in mice that had lost expression of the TP53 tumor suppressor a situation that mirrors up to 60 percent of human pancreatic cancer cases tumors escape the dependency on EGFR for initiation and continued growth of pancreatic cancer, Dr. Crawford says.
View post:
Cancer-causing gene alone doesn't trigger pancreatic cancer, Mayo-led study finds
Recommendation and review posted by Bethany Smith
Researchers find 2 gene mutations drive adrenal cancer
Public release date: 10-Sep-2012 [ | E-mail | Share ]
Contact: Nicole Fawcett nfawcett@umich.edu 734-764-2220 University of Michigan Health System
This press release is available in Portuguese.
ANN ARBOR, Mich. Two different genetic mutations cooperate to induce adrenal cancer, according to a new study from researchers at the University of Michigan Comprehensive Cancer Center and University of Sao Paulo in Brazil.
The finding provides new clues to this rare and deadly cancer type, and researchers hope it will lead to better treatments by targeting both mutations.
About 600 Americans are diagnosed with adrenal cancer per year. It is typically diagnosed in late stages when there is nearly no chance of survival beyond five years.
"Because adrenal cancer is so rare, it has been challenging to find enough patients who can provide tissue samples for research. Only through collaboration can we do this," says senior study author Gary Hammer, M.D., Ph.D., the Millie Schembechler Professor of Adrenal Cancer at the University of Michigan Comprehensive Cancer Center.
The partnership between U-M and Sao Paulo has allowed researchers to collect tissue samples from 118 people with benign or cancerous adrenal tumors.
"Our goal is to understand these tumors and the genes that are critical lynchpins so that we can develop treatments that extend patients' lives," Hammer says.
By studying both benign and cancerous adrenal tissue samples, the researchers found aberrations in two genetic pathways: beta-catenin and insulin-like growth factor 2, or IGF-2. The benign tumors had a high percentage of mutations in beta-catenin, but IGF2 up-regulation was rare. On the other hand, most of the adrenal cancers exhibited IGF-2 up-regulation. Cancers that also had beta-catenin mutation were associated with high-grade disease and worse survival, compared to tumors with only IGF-2 up-regulation.
The rest is here:
Researchers find 2 gene mutations drive adrenal cancer
Recommendation and review posted by Bethany Smith
Two gene mutations drive adrenal cancer
ScienceDaily (Sep. 10, 2012) Two different genetic mutations cooperate to induce adrenal cancer, according to a new study from researchers at the University of Michigan Comprehensive Cancer Center and University of Sao Paulo in Brazil.
The finding provides new clues to this rare and deadly cancer type, and researchers hope it will lead to better treatments by targeting both mutations.
About 600 Americans are diagnosed with adrenal cancer per year. It is typically diagnosed in late stages when there is nearly no chance of survival beyond five years.
"Because adrenal cancer is so rare, it has been challenging to find enough patients who can provide tissue samples for research. Only through collaboration can we do this," says senior study author Gary Hammer, M.D., Ph.D., the Millie Schembechler Professor of Adrenal Cancer at the University of Michigan Comprehensive Cancer Center.
The partnership between U-M and Sao Paulo has allowed researchers to collect tissue samples from 118 people with benign or cancerous adrenal tumors.
"Our goal is to understand these tumors and the genes that are critical lynchpins so that we can develop treatments that extend patients' lives," Hammer says.
By studying both benign and cancerous adrenal tissue samples, the researchers found aberrations in two genetic pathways: beta-catenin and insulin-like growth factor 2, or IGF-2. The benign tumors had a high percentage of mutations in beta-catenin, but IGF2 up-regulation was rare. On the other hand, most of the adrenal cancers exhibited IGF-2 up-regulation. Cancers that also had beta-catenin mutation were associated with high-grade disease and worse survival, compared to tumors with only IGF-2 up-regulation.
The researchers additionally tested this finding experimentally by inducing individual or combined mutations in beta-catenin and IGF-2 in the mouse adrenal. The mice developed cancer only when both mutations were present.
Results of the study appear in the September issue of the American Journal of Pathology.
The next step is to develop treatments that block both beta-catenin and IGF2.
Read more:
Two gene mutations drive adrenal cancer
Recommendation and review posted by Bethany Smith
Cancer-causing gene alone doesn’t trigger pancreatic cancer, research finds
ScienceDaily (Sep. 10, 2012) More than a cancer-causing gene is needed to trigger pancreatic cancer, a study led by Mayo Clinic has found. A second factor creates a "perfect storm" that allows tumors to form, the researchers say. The study, published in the Sept. 10 issue of Cancer Cell, overturns the current belief that a mutation in the KRAS oncogene is enough to initiate pancreatic cancer and unrestrained cell growth.
The findings uncover critical clues on how pancreatic cancer develops and why few patients benefit from current therapies. The findings also provide ideas about how to improve treatment and prevention of pancreatic cancer.
The research team, led by Howard C. Crawford, Ph.D., a cancer biologist at Mayo Clinic's campus in Florida, and Jens Siveke, M.D., at Technical University in Munich, Germany, found that for pancreatic cancer to form, mutated KRAS must recruit a second player: the epidermal growth factor receptor, or EGFR.A third genetic participant known as Trp53 makes pancreatic tumors very difficult to treat, the study showed.
The scientists also found that EGFR was required in pancreatic cancer initiated by pancreatic inflammation known as pancreatitis.
"We believe the perfect storm needed to trigger pancreatic cancer include KRAS mutations and inflammation in the organ, which then work synergistically to turn on EGFR," says Dr. Crawford.
"The bottom line is, without EGFR, tumors don't form -- and that was never known before this study," he says. "We also think that inflammation in the pancreas has a big impact on turning on EGFR."
The researchers discovered that when they blocked EGFR activity, the mice studied were protected against developing chronic pancreatitis and pancreatic cancer.
They further found that in mice that had lost expression of the TP53 tumor suppressor -- a situation that mirrors up to 60 percent of human pancreatic cancer cases -- tumors escape the dependency on EGFR for initiation and continued growth of pancreatic cancer, Dr. Crawford says.
Pancreatic cancer is a highly lethal disease; no drug has been able to target the mutant KRAS protein. The study suggests some patients, such as those with chronic pancreatitis, may be good candidates for treatment with EGFR inhibitors to fight or prevent pancreatic cancer, Dr. Crawford says.
"The clinical implications of this study are exciting. It suggests that pancreatic cancer patients with normal p53 activity, as well as patients with chronic pancreatitis, may be good candidates for treatment with EGFR inhibitors," Dr. Crawford says.
See the rest here:
Cancer-causing gene alone doesn’t trigger pancreatic cancer, research finds
Recommendation and review posted by Bethany Smith
Mandatory GM Labeling Would Require Major Change
CPG manufacturers may be on the cusp of monumental change as voters in California contemplate a hotly contested ballot initiative to require labeling of genetically modified foods.
Food marketers will face tough choices should the measure pass, as about 70% of processed foods sold in supermarkets contain GM ingredients like corn and soy. Some estimate that 100,000 or more foods sold in California contain some level of GE ingredients and would therefore be affected.
The mandate would be limited to the Golden State, but the implications for companies that choose not to move away from GM ingredients in advance of the July 1, 2014, deadline could be as far-reaching as consumer awareness spreads.
While the government deems genetically modified organisms safe, Californians want to judge for themselves. A Pepperdine University poll found that if the election were held last month, Californians would pass the proposition by a 3-1 margin.
To avoid the partially produced with genetic engineering label and possible consumer backlash, suppliers will likely reformulate product with more costly non-GE foods or organic ingredients, just as theyve done in countries where genetic modification disclosure is required.
Read more: Prop 37 Battle Rages in California
A recent study commissioned by the No on 37, Stop the Deceptive Food Labeling Scheme campaign, of which the Grocery Manufacturers Association is a chief sponsor, bears this out.
It projects that reformulations to non-GE and organic ingredients, which by law cannot be genetically modified, will be the most likely course taken by food producers.
Read more: California GMO Bill Is Top Priority for GMA
Retailers might also adjust their sourcing policies to gain consumer favor by incorporating more organic foods and those that have been verified under the Non-GMO Project and labeled with its seal.
See the original post:
Mandatory GM Labeling Would Require Major Change
Recommendation and review posted by Bethany Smith
GEN reports on ocular therapeutics targeting the retina
Public release date: 10-Sep-2012 [ | E-mail | Share ]
Contact: John Sterling jsterling@genengnews.com 914-740-2196 Mary Ann Liebert, Inc./Genetic Engineering News
New Rochelle, NY, September 10, 2012-- Therapies for retinal diseases are expected to overtake those for glaucoma by 2014, reports Genetic Engineering & Biotechnology News (GEN). Because current retinal disease treatments only improve vision for six to eight weeks, there is a critical need for new remedies, according to a recent issue of GEN.
"As increasing numbers of baby-boomers continue to grow older, many will have to deal with eye diseases such as age-related macular degeneration," said John Sterling, Editor-in-Chief of GEN. "Some estimates put the current AMD and diabetic retinopathy drug segment of the market at $3 billion, and this is expected to increase to about $5 billion in two years."
Standard therapy has been Genentech's VEGF inhibitors Lucentis and the off-label use of Avastin. Regeneron, in collaboration with Bayer HealthCare, is challenging these drugs with a similar VEGF inhibitor, Eyela. The FDA approved the drug last November for wet AMD.
In another approach, Acucela is in Phase II trials using visual cycle modulators to lighten the metabolic load on the retina by reducing the activity of the rod visual system. This protects the retina from light damage, improves retinal vasculature, and reduces the accumulation of A2E and other retinal-related toxic by-products.
GlaxoSmithKline has two drugs in Phase II trials for ocular therapy: darapladib, an oral Lp-PLA2 inhibitor for diabetic macular edema, and Votrient, a multi-kinase angiogenesis inhibitor in eye drop form for AMD. Early-stage work also is under way for neovascular AMD, dry AMD, diabetic retinopathy, diabetic macula edema, uveitis, and glaucoma, as well as for technologies for drug delivery.
###
Other companies covered in the GEN article include Acucela, pSiveda, Sanofi, NeuroTech, QLT, Applied Genetic Technologies, RetroSense Therapeutics, Aerie Pharmaceuticals, Kowa Pharmaceuticals America, Novartis, Senju Pharmaceutical, Can-Fite Biopharma, Inotek Pharmaceuticals, Otsuka Pharmaceutical, and Santen Pharmaceutical.
For a copy of the September 1 issue of GEN, please call (914) 740-2146, or email: pbartell@genengnews.com
Go here to read the rest:
GEN reports on ocular therapeutics targeting the retina
Recommendation and review posted by Bethany Smith
New genetic mechanism for controlling blood cell development and blood vessel integrity found
ScienceDaily (Sep. 10, 2012) The protein GATA2 is known as a "master regulator" of blood cell development. When a mutation occurs in the gene that makes GATA2, serious blood diseases such as acute myeloid leukemia can result.
Zooming in on the GATA2 gene, UW-Madison researchers and their collaborators at the National Institutes of Health (NIH) have discovered unexpectedly that a small DNA sequence drives this powerful master regulator. The sequence plays an essential role in controlling GATA2 production and generating self-renewing blood stem cells responsible for the earliest steps in the development of blood cells of all kinds -- red cells to transport oxygen and white cells to fight infection.
The researchers also found that the DNA sequence, which they call the +9.5 GATA2 switch site, ensures that blood vessels function properly to prevent hemorrhaging. Until now, GATA2 had not been implicated in blood vessel integrity.
The study appears in The Journal of Clinical Investigation (online Sept. 10, 2012).
Although the study was performed in mice, it should have significant clinical relevance, particularly to physicians and scientists aiming to understand certain types of leukemia and related disorders involving disruptions in the blood and immune systems. The research indicates that downstream genes are impacted when the switch site is altered, leading to abnormal development of the adult blood system, says senior author Dr. Emery Bresnick, professor of cell and regenerative biology at the School of Medicine and Public Health.
"There's every reason to believe that we can use these findings as a foundation to discover key factors and signals that can be modulated therapeutically for the treatment of specific blood and blood vessel disorders," he says.
Bresnick has studied GATA proteins for more than a decade. Among other things, his team discovered five "hot spots" on the GATA2 gene where special activity involving both GATA1 and GATA2 appeared to be taking place. They named these GATA switch sites. Over the last four years, the group has focused on their third GATA2 switch site, +9.5, a small sequence of about 25 base pairs located in a region of the gene called the intron.
"While introns can contain regulatory sequences that control gene activity, until now we and others have been unable to find the mechanisms that control the GATA2 gene, despite years of studying this problem," Bresnick says.
Senior scientist Dr. Kirby Johnson from the Bresnick laboratory led experimental efforts to genetically engineer and breed mice in which the site was knocked out, and then studied the consequences of the mutation.
Without the sequence, the embryos died on day 14, but before that they developed massive hemorrhaging. Closer analysis showed that large veins in the embryo leaked embryonic red blood cells.
Visit link:
New genetic mechanism for controlling blood cell development and blood vessel integrity found
Recommendation and review posted by Bethany Smith
Arroyo undergoes 4th stem cell treatment
By Leila B. Salaverria Philippine Daily Inquirer
Former President and now Pampanga Rep. Gloria Macapagal Arroyo: Stem cell treatment
MANILA, PhilippinesLike her predecessor, former President and Pampanga lawmaker Gloria Macapagal Arroyo has turned to stem cell therapy in an effort to improve her health.
Arroyo said in her official Twitter account that she would have her fourth stem cell intravenous treatment with her alternative medicine doctor on Monday.
Arroyo said her treatment would involve cultured stem cells, and it would be much more modest in price than the one coming from sheep or ones own body.
A close friend and ally of Arroyo, Quezon Representative Danilo Suarez, confirmed that the President has started stem cell therapy, and that she told him that the stem cells she has been using did not come from lamb placenta, and was the less costly form of stem cell treatment.
If you have a lot of health problems, you will try such things, Suarez said on Sunday.
Suarez said he has even filed a resolution to investigate the practice of stem cell treatments in the country, as well as the claims being made about it, considering that it has been gaining popularity.
The public needs to be better informed about it. It might have setbacks that we need to know about, he said.
The therapy involves the use of fresh cells, which are injected into the body to regenerate cells to treat illnesses or reverse aging.
See the original post here:
Arroyo undergoes 4th stem cell treatment
Recommendation and review posted by simmons
Surprising methods heal wounded troops
(AP) BOSTON - Scientists are growing ears, bone and skin in the lab, and doctors are planning more face transplants and other extreme plastic surgeries. Around the country, the most advanced medical tools that exist are now being deployed to help America's newest veterans and wounded troops.
Much of this comes from taxpayer-funded research. Four years ago, the federal government created AFIRM, the Armed Forces Institute of Regenerative Medicine, a network of top hospitals and universities, and gave $300 million in grants to spur new treatments using cell science and advanced plastic surgery.
"The whole idea is to bring all these researchers together to develop these great technologies that were in early science to eventually be ready for the troops," said AFIRM's recently retired director, Terry Irgens.
Now those who served are coming home, and projects that once had been languishing in labs are making strides and starting to move into clinics.
Armed Forces Institute of Regenerative Medicine Veterans Affairs: Research & Development Operation Mend
Strang is among those benefiting. The 28-year-old Marine sergeant from Pittsburgh lost half of a thigh muscle to shrapnel, leaving too little to stabilize his gait. "My knee would buckle and I'd fall over," he said.
Now, after an experimental treatment at the University of Pittsburgh Medical Center, "I'm able to run a little bit" and play a light football game with friends, he said. "It's been a huge improvement."
It's one example of the "new medicine" in the works for troops. The Associated Press conducted more than a dozen interviews and reviewed the latest medical research to measure the progress and extent of novel treatments under way for wounded warriors. The results point to some surprising feats of surgery and bioengineering.
Up to a thousand troops might need an ear, and prosthetics are not a great solution. A rod or other fastener is required to attach them to the head. They don't look or feel natural and they wear out every couple of years. A matching ear grown from a patient's own cells would be a huge improvement.
"People have been working on this for 20 years" but haven't been able to overcome obstacles to making it practical, said Cathryn Sundback, director of the tissue engineering lab at Massachusetts General Hospital.
See the original post here:
Surprising methods heal wounded troops
Recommendation and review posted by sam
Arroyo undergoes 4th stem cell treatment
By Leila B. Salaverria Philippine Daily Inquirer
Former President and now Pampanga Rep. Gloria Macapagal Arroyo: Stem cell treatment
MANILA, PhilippinesLike her predecessor, former President and Pampanga lawmaker Gloria Macapagal Arroyo has turned to stem cell therapy in an effort to improve her health.
Arroyo said in her official Twitter account that she would have her fourth stem cell intravenous treatment with her alternative medicine doctor on Monday.
Arroyo said her treatment would involve cultured stem cells, and it would be much more modest in price than the one coming from sheep or ones own body.
A close friend and ally of Arroyo, Quezon Representative Danilo Suarez, confirmed that the President has started stem cell therapy, and that she told him that the stem cells she has been using did not come from lamb placenta, and was the less costly form of stem cell treatment.
If you have a lot of health problems, you will try such things, Suarez said on Sunday.
Suarez said he has even filed a resolution to investigate the practice of stem cell treatments in the country, as well as the claims being made about it, considering that it has been gaining popularity.
The public needs to be better informed about it. It might have setbacks that we need to know about, he said.
The therapy involves the use of fresh cells, which are injected into the body to regenerate cells to treat illnesses or reverse aging.
Read this article:
Arroyo undergoes 4th stem cell treatment
Recommendation and review posted by Bethany Smith
Large lung cancer study shows potential for more targeted therapies
Public release date: 9-Sep-2012 [ | E-mail | Share ]
Contact: Julia Evangelou Strait straitj@wustl.edu 314-286-0141 Washington University School of Medicine
A nationwide consortium of scientists has reported the first comprehensive genetic analysis of squamous cell carcinoma of the lung, a common type of lung cancer responsible for about 400,000 deaths each year.
"We found that almost 75 percent of the patients' cancers have mutations that can be targeted with existing drugs -- drugs that are available commercially or for clinical trials," says one of the lead investigators, Ramaswamy Govindan, MD, an oncologist at Washington University School of Medicine in St. Louis and co-chair of the lung cancer group of The Cancer Genome Atlas.
The research appears online Sept. 9 in Nature.
The Cancer Genome Atlas project combines efforts of the nation's leading genetic sequencing centers, including The Genome Institute at Washington University, to describe the genetics of common tumors with the goal of improving prevention, detection and treatment. The Cancer Genome Atlas is supported by the National Cancer Institute and the National Human Genome Research Institute, both parts of the National Institutes of Health (NIH).
The other lung cancer co-chairs are the study's senior author Matthew Meyerson, MD, PhD, of the Broad Institute of Massachusetts Institute of Technology and Harvard University, and Stephen Baylin, MD, of Johns Hopkins University.
The study examined the tumors and normal tissue of 178 patients with lung squamous cell carcinoma. The investigators found recurring mutations common to many patients in 18 genes. And almost all of the tumors showed mutations in a gene called TP53, known for its role in repairing damaged DNA.
Interestingly, the researchers noted that lung squamous cell carcinoma shares many mutations with head and neck squamous cell carcinomas, supporting the emerging body of evidence that cancers may be more appropriately classified by their genetics rather than the primary organ they affect.
"We clearly see mutations in lung cancer that we see in other human cancers," says Richard K. Wilson, PhD, director of The Genome Institute at Washington University. "This reinforces something that we've been seeing in a lot of our cancer genomics work. It's really less about what type of tissue the tumor arises in lung, breast, skin, prostate and more about what genes and pathways are affected."
Read more:
Large lung cancer study shows potential for more targeted therapies
Recommendation and review posted by Bethany Smith
SCI-TECH: Autism gene found by UCSD researchers
A genetic cause for a rare form of epilepsy-associated autism has been identified by UC San Diego and Yale scientists.
Moreover, symptoms of the newly discovered form have been reversed in mouse models by altering diet. This gives rise to the possibility that similar treatment might help people, the researchers said.The study was published online Thursday in the journal Science.
Researchers led by Gaia Novarino and Joseph G. Gleeson of UCSD studied two families, one of Egyptian descent and another of Turkish origin. They examined the genome of patients and healthy relatives for exons, gene sequences that code for proteins. The researchers found that patients shared an exon mutation on a gene called BCKDK. The mutant gene is recessive, meaning that it must be inherited from both mother and father to manifest.
Moreover, the researchers found that the mutation caused patients to produce abnormally low levels of certain types of amino acids, the building blocks of proteins. They were able to boost levels of these amino acids to normal with a nutritional supplement from a health food store. Research is now ongoing as to whether this supplementation will reduce symptoms of epilepsy and autism in these patients.
Those who might be helped are only a small fraction of people with autism, Novarino said in an Tuesday interview. Those without the metabolic defect wouldn't benefit from the supplementation.
The study illustrates how scientists have become more sophisticated in using knowledge of the human genome to crack the puzzle of previously intractable diseases.The genome is the complete set of hereditary information encoded in DNA.
Narrowing the search
The vast majority of DNA does not code for proteins, the body's workhorse molecules. This "non-coding" DNA was ignored in the new method of DNA analysis, called "whole exome" sequencing, which looks only at the exons. An advantage of whole exome sequencing is that it focuses exclusively on proteins, which are altered or missing in genetic diseases.
Whole exome sequencing can find previously undiscovered genetic diseases, according to another study performed by some of the same UCSD researchers. They examined 118 patients diagnosed with neurological disorders who had no known genetic disease causes. In addition to the newly discovered genetic causes, in about 10 percent of cases the researchers even found a known disease-causing gene that had previously escaped detection.
That study was published in June in Science Translational Medicine, a journal devoted to getting research discoveries into the hands of doctors more quickly.
Visit link:
SCI-TECH: Autism gene found by UCSD researchers
Recommendation and review posted by Bethany Smith